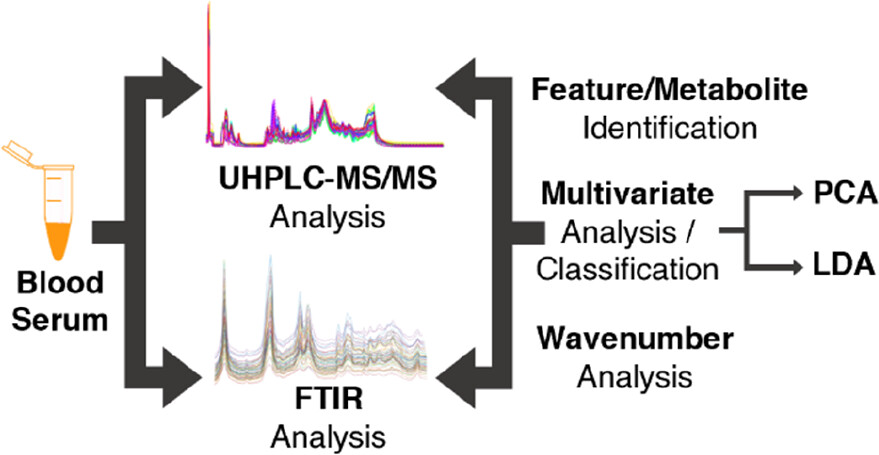
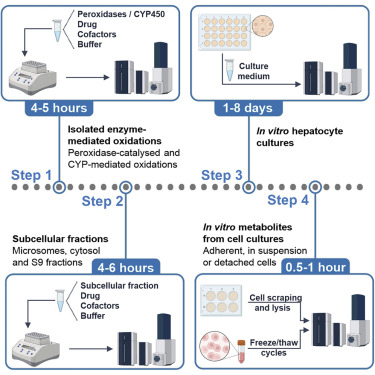
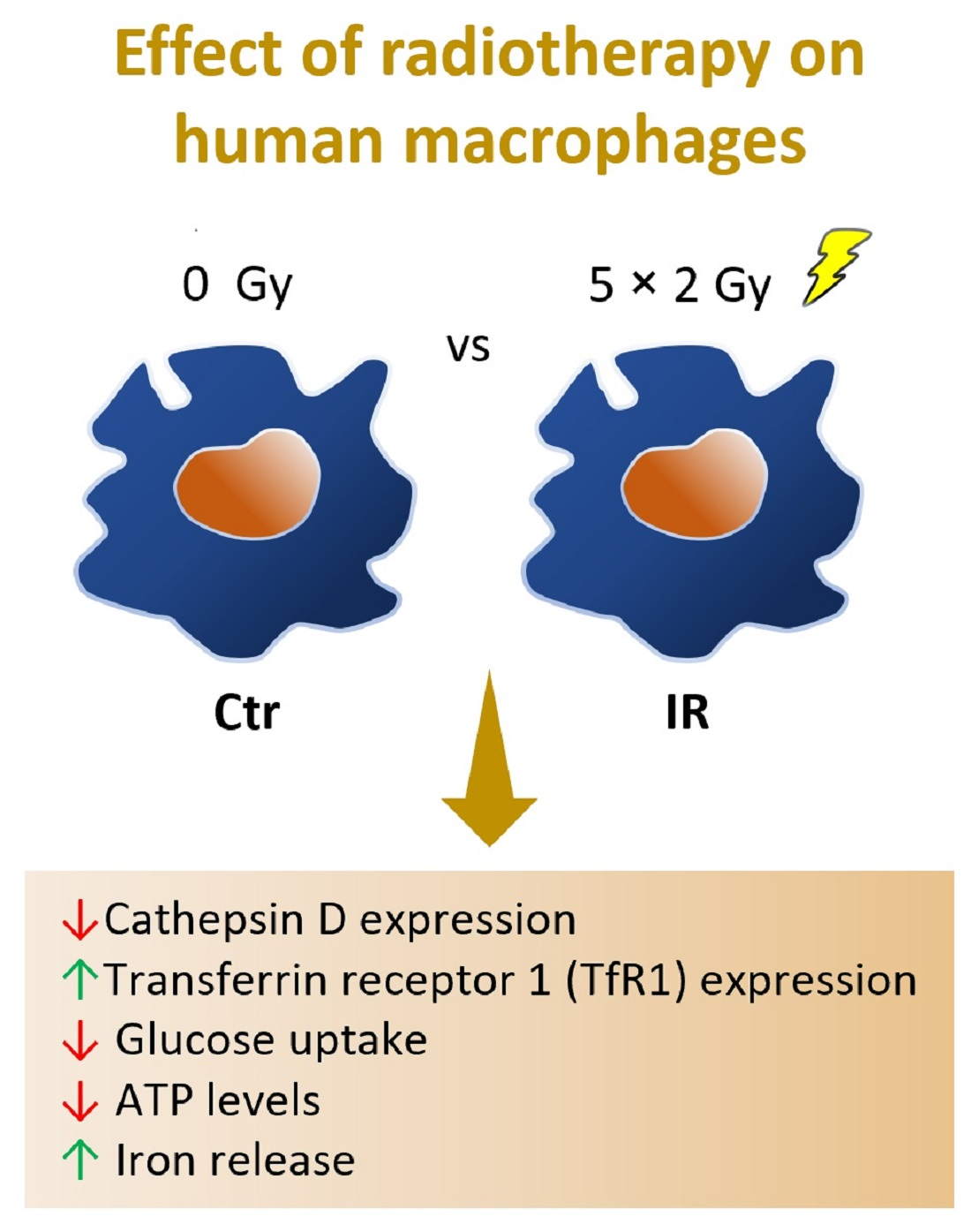
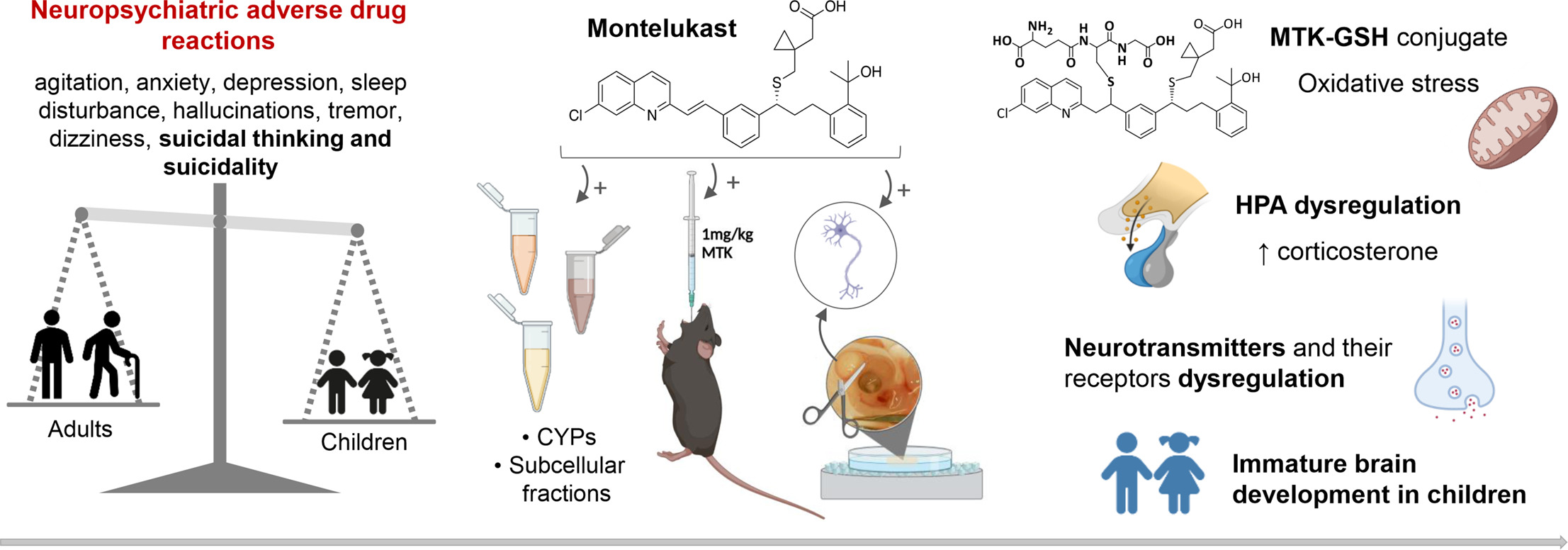
Macrophage
Resistance to Ionizing Radiation Exposure Is Accompanied by Decreased Cathepsin
D and Increased Transferrin Receptor 1 Expression
Resistance to treatment, particularly to
radiotherapy, is still a major clinical problem in cancer management.
Macrophages are abundant immune cells at the tumor microenvironment,
being exposed to ionizing radiation during cancer radiotherapy.
Considering the role of macrophages in tumor progression and therapy
outcome, it is crucial to investigate their response to clinically
relevant ionizing radiation doses for the design of new strategies to
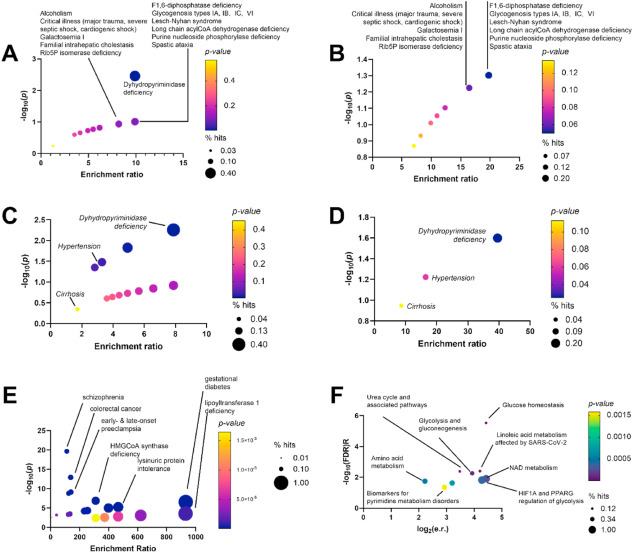
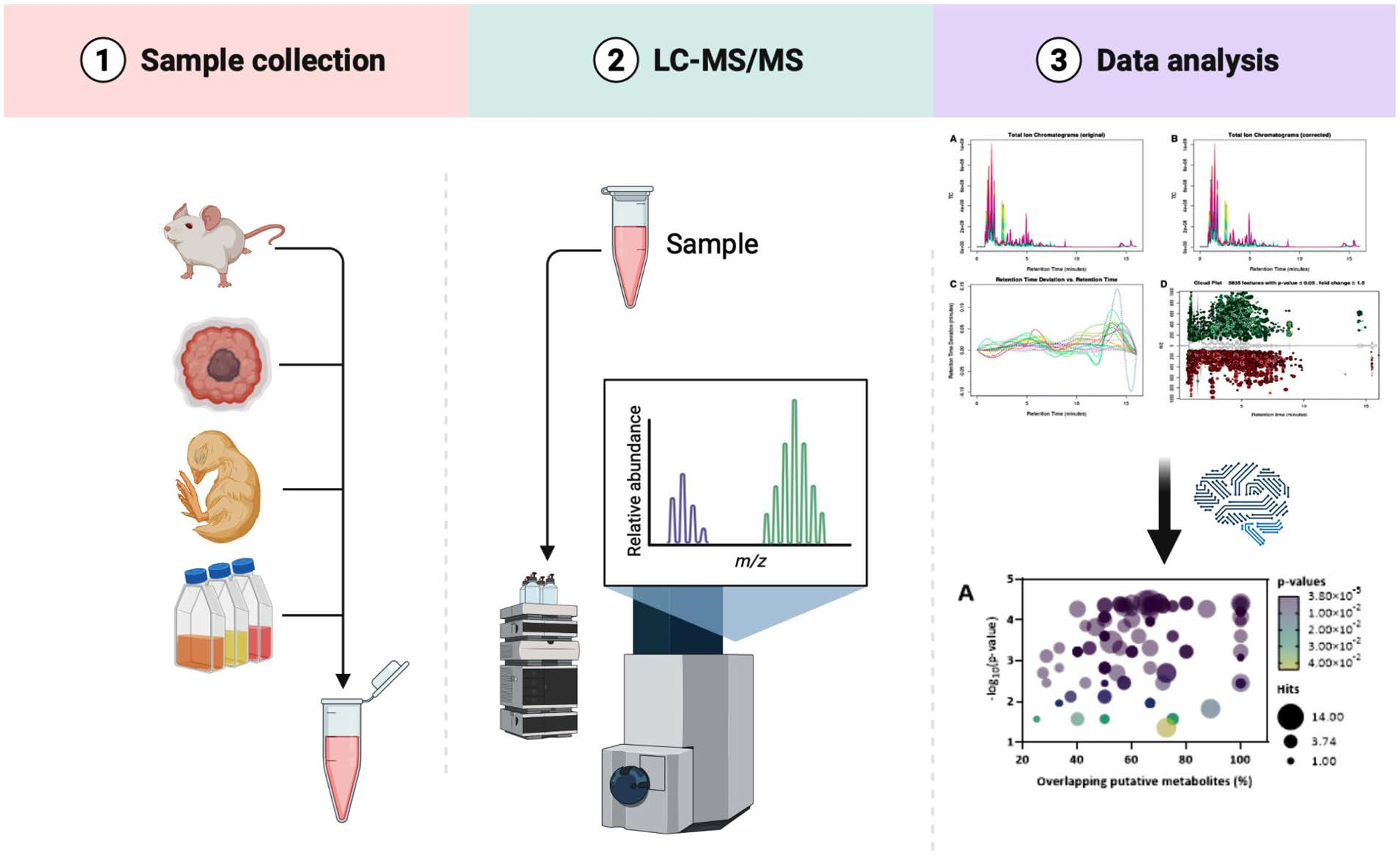
overcome tumor radio resistance. In this work, we have used a proteomic
approach to evaluate the expression profile of irradiated versus
non-irradiated macrophages. This analysis, supported by validation using
cell-based assays, led to the identification of two main deregulated
targets, cathepsin D and transferrin receptor 1, in irradiated
macrophages. Investigating macrophage response to ionizing radiation
could lead to the identification of deregulated pathways and molecular
players that can be targeted to overcome tumor radio resistance.
Learn More
Pinto AT*, Machado AB, Osório H, Pinto ML,
Vitorino R, Justino GC, Santa C, Castro F, Cruz T, Lima J, Cardoso AP,
Figueira R, Monteiro A, Marques M, Manadas B, Pauwels J, Gevaert K, Mareel M,
Rocha S, Duarte T, Oliveira, MJ. Macrophage Resistance
to Ionizing Radiation Exposure Is Accompanied by Decreased Cathepsin D and
Increased Transferrin Receptor 1 Expression. Cancers2023 15(1):270.